Fritz Haber and Carl Bosch revolutionized the agricultural industry when they developed a process to synthesize ammonia. More than a century later, ammonia finds itself at the center of the green fuel revolution. But cleanly producing low-cost ammonia at the scale necessary to meet the world’s energy needs will require innovations in renewable energy and ammonia production processes.
The Haber-Bosch process combines one nitrogen atom and three hydrogen atoms to form ammonia. The nitrogen is harvested from the air all around us, while the hydrogen is produced using primarily steam methane reformation or coal gasification (see this article for more information). Ammonia’s role as a nitrogen carrier makes it commonly used in fertilizers today. With annual global demand for ammonia exceeding 150 million metric tons, ammonia production today generates more than 75 billion USD in revenue every year. And the demand is expected to grow!
Unfortunately, it takes approximately 2% of the world’s energy to produce this much ammonia, and almost all of this production uses natural gas or coal as the energy source. Consequently, ammonia production is responsible for approximately 2% of the world’s annual CO2e emissions. That’s more than 500 million metric tons of CO2e emissions every year! Each metric ton of ammonia produced using natural gas results in 2.6 metric tons of CO2e emissions while each metric ton produced using coal results in 6.1 metric tons of CO2e emissions. Producing ammonia using renewable energy sources is a crucial step toward making a tangible impact on our carbon footprint in both the agricultural sphere today and the fuel sector in the future. 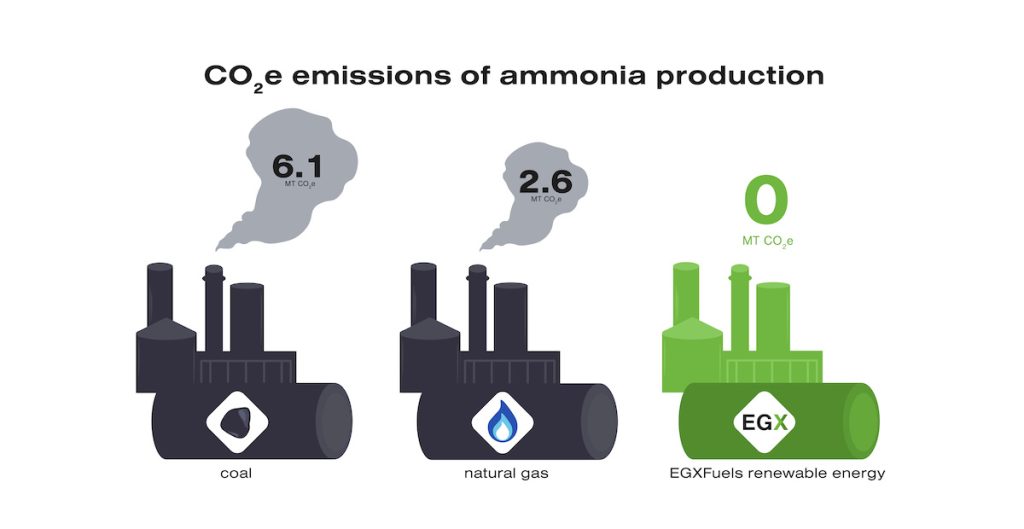
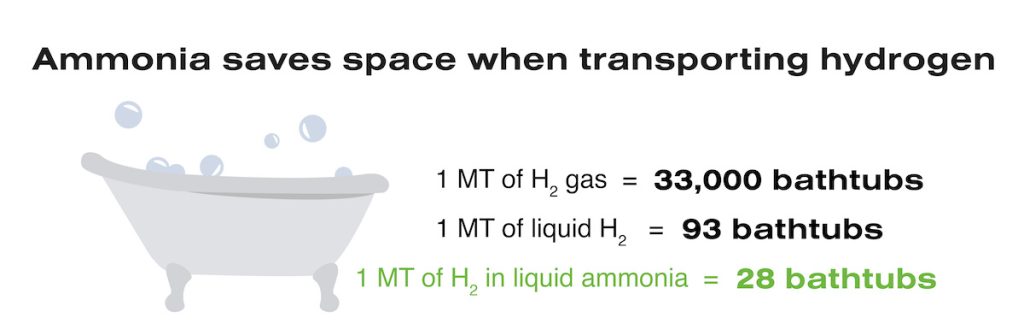
In addition to its use as a nitrogen carrier in the agricultural industry, ammonia also serves as an efficient hydrogen carrier in the fuel sector. It’s not an easy feat to transport hydrogen for use in fuel cells or as a combustible fuel. That’s because at normal atmospheric temperature and pressure, one metric ton of hydrogen gas takes up more than 350,000 cubic feet of space, which is approximately 33,000 bathtubs! To efficiently ship hydrogen, there are two primary methods to reduce its volume.
First, we can turn hydrogen into a liquid by cooling it to -423°F (extreme cooling) or we can acheive the same density by pressurizing it more than 25,000 psi (extreme pressurization). Both processes achieve the same volume of 993 cubic feet (the equivalent of 93 bathtubs); however, both processes are prohibitively energy intensive.
Alternatively, we can combine hydrogen with nitrogen to create ammonia for shipping. Fortunately, ammonia reaches liquid state by cooling it to only -28°F or pressurizing it to 300 psi. In its liquid state, 5.63 metric tons of ammonia, which contains 1 metric ton of hydrogen, only takes up 292 cubic feet (28 bathtubs). Using only moderate cooling or pressurization, we can use ammonia to transport 1200 metric tons of hydrogen in the space it takes to ship one metric of hydrogen alone!
Each EGXFuels production site is forecast to produce more than 350,000 metric tons of green hydrogen every year. With the help of our electrically driven green ammonia production process, we will be able to provide renewable energy to locations across the globe. Today, renewable energy is only locally available through an electrical grid and ammonia is primarily used in the fertilizer sector. We envision a world where renewable energy is accessible to everyone, and ammonia is the key to making this a reality.

the future of fuel
Corporate Office
136 4th St N, Ste 212,
St. Petersburg, Florida 33701
Engineering Office
16225 Park 10 Place, Suite 500,
Houston, Texas 77084
Copyright © 2024 EGXFuels. All rights reserved.

